On July 4, 2021, China’s National Medical Products Administration (NMPA) and the China National Intellectual Property Administration (CNIPA) released details on the new implementation measures for early dispute resolution mechanisms for drug patents (“Patent Linkage”), effective July 4, 2021.
Below is a summary highlighting key provisions and changes from the draft measures.
Creation of an “Orange Book” List
A new public registration platform has been created that lists “Patent Information” for all drugs marketed in China (similar to the US “Orange Book”). Drugs must be approved in China in order to be listed, and unlisted drugs cannot use these new “Patent Linkage” mechanisms. What “Patent Information” needs to be provided? At least the drug name, drug dosage form, specification, patent number, patent type, patent status, patentee, patent licensee, marketing authorization holder, patent grant and expiry date, contact information, and how the patent claims correspond to the drug. Users will be able to go to the online system themselves and update information as necessary.
Applicants and marketing authorization holders are responsible for the accuracy of the information posted. The registration information should be consistent with other official documents. For example, the Registration Information should be consistent with what’s in the patent register, patent gazette, and drug registration certificate. Medical uses (composition for use claims) should be consistent with the indications for the drug listed on the approved drug label. Patent Protection Scope should cover the technical solutions of the approved drug. Any modifications to the information must be explained.
Deadlines for “Orange Book” List Submissions
Drug holder has 30 days after receiving marketing authorization of a drug to register the information for the drug in the “Orange Book.” All updates need to be submitted within 30 days of a status change.
Types of Patents that can be Listed
|
Type of Drug |
Listable Patents |
|
Small Molecules |
Compound of API (active pharmaceutical ingredient) |
|
Composition/Formulation of API |
|
|
Medical use[1] of API |
|
|
Biologics |
Sequence structure of the biologic |
|
Medical use of the biologic |
|
|
Traditional Chinese Medicine (TCM) |
Composition comprising TCM |
|
Ingredients extracted from TCM |
|
|
Medical use of TCM |
Certifying Patent Status by Generic Drug Applicants
Similar to US requirements under Hatch Waxman, generic small molecule drug applicants in China must “certify” the patent status of the innovator drug they are seeking to sell by making one of the below four statements and providing supporting evidence.
|
Category |
Certifying declaration by generic applicant |
Approval timeline |
|
Category I |
No patent relating to the original drug is listed |
Immediately |
|
Category II |
The listed patent has expired or was invalidated, or the generic company has obtained a license from the patent holder |
Immediately |
|
Category III |
There is a listed patent and the generic drug will not enter the market until the listed patent expires |
Immediately, but must wait until expiration of patent to enter market |
|
Category IV |
The generic drug applicant believes the listed patent should be invalid or not infringed |
Innovator company has 45 days after publication of certification to file suit in the People’s Court or oppose with CNIPA; |
As show above, if there is no enforceable patent blocking the generic company, then the application will be approved after the drug application passed the technical review. However, if there is a granted patent on the list that is still in force, the generic company needs to certify that it believes the patent is invalid or not infringed, almost mirroring the Paragraph IV Certifications under the FDA in the US.
The Marketing Authorization Holder’s Response
A welcomed change from the draft implementation measures is that within 10 days of the acceptance by the NMPA of the generic drug Applicant’s declaration, the generic drug applicant must notify the marketing authorization holder[2] of the declaration made at the NMPA. If statement says that the product does not fall within the patent, the statement should include comparison table showing the generic technical solution compared to the patent claims. The notification should be sent as a hard copy and an emailed soft copy.
Upon receiving notice, the Patentee has 45 days to either file suit at the People’s Court or request an administrative ruling at the CNIPA (see below), but not both. The innovator has 15 days to notify the National Drug Review Institution (NDRI) and the generic drug Applicant about the suit. Once a judgment is received, the innovator has 10 days to notify the NMDA of the decision.
In small molecule cases, the NMPA will continue its technical review but will stay its approval decision for a one-shot waiting period (9 months) to allow the litigation/opposition to conclude. Once a decision is made, the NMPA will based its decision accordingly on the outcome. If the patent is upheld and infringement would occur, the NMPA will wait until patent expiry to approve the generic. Otherwise, it will approve immediately. See Table 2 for more details.
Table 2
|
Outcome of the People’s Court / CNIPA |
Approval Timeline |
|
Generic drug does NOT fall within the patent |
Normal administrative procedure for review |
|
Parties settle the case |
|
|
Patent is invalidated |
|
|
No decision issued within the 9-month stay period |
|
|
Generic DOES falls within the scope of the patent |
Initial administrative review begins right before the patent expiration date |
If an initial decision changes, for example a CNIPA decision is overturned by the Court, the parties later agree to license or settle, or the patentee withdraws the suit, the generic drug applicant can re-apply to NMDA for approval again.
The first small molecule generic drug applicant to successfully invalidate a listed drug’s patent and get approval can enjoy its own 6-months data exclusivity and 12-month market exclusivity (against other generics), which is more “generous” than the US’s 180-day exclusivity period for a similar provision. This exclusivity period can only last up to the innovator patent’s expiration date.
Biologics and Traditional Chinese Medicine
For biologics and traditional Chinese medicine, the NMPA will not stay approval decisions but will just proceed with the approval process based on recommendations of the NDRI. If a litigation concludes favourably (for the patentee) before the generic approval has been made, the NMPA can still continue the approval process but will indicate that such drug should not enter the market until the listed patent expires.
The rules state that if the People’s Court or the CNIPA has not issued a decision by the 9‑month stay period, the generic market application will be approved. It is difficult to tell at this point whether the Chinese courts and the CNIPA can move through these litigations quickly enough in order to hand down a decision within the 9-month period. Earlier drafts of this provision provided both 9 months and 24 months. Both are in stark contrast to the US, where a 30-month stay is granted. It remains to be seen how this will play out. Although it is true that Chinese litigations tend to be much shorter (by law, the time limit to hear first instance civil cases is 6 months), sometimes complex IP cases can still drag on for 1-2 years.
The silver lining in all of this is that, even if the generic drug is approved by the NMPA (and the patent linkage is essentially “broken”), the innovator can still sue the generic company under Chinese Patent Law and may still be able to recover damages, obtain injunctions, etc. However, the NMPA will not withdraw any approvals even if infringement is ultimately found.
CNIPA will Handle Patent Linkage Administrative Disputes
CNIPA has established a dedicated Patent Linkage Committee to handle the administrative disputes under this new mechanism. On July 4, 2021 (same day) CNIPA issued a set of procedures rules (effective immediately) governing these patent linkage administrative cases.
General Requirements
This new “Patent Linkage” Committee will only adjudicate requests pertaining to whether a generic drug (applying for approval) infringes a listed patent (“Request”). Only a generic drug applicant, patent owner, licensee, or marketing authorization holder may file such a Request, though there are additional rules governing who has the right to file a Request first[3]. For a Request to be granted by CNIPA, there must be no other pending court proceedings between the two parties on the same drug.
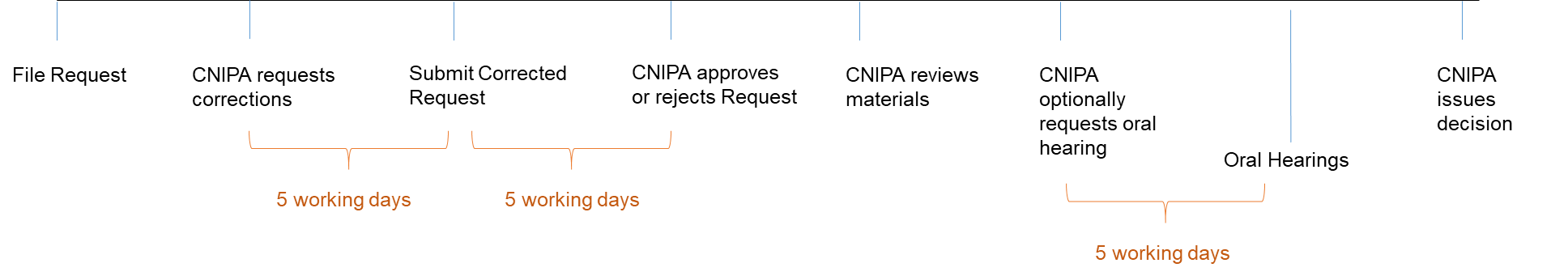
Short Timelines
Timelines are relatively short for this procedure. After a Request is submitted, the CNIPA will review and notify the requester if there are any issues with the submission. Requesters only have five working days to correct any issues in the Request submission. Likewise, CNIPA will respond within 5 working days indicating whether a Request is accepted. Once the Request is accepted, CNIPA will review the Request plus accompanying materials and will initiate an oral hearing if necessary, giving the parties at least 5-working days’ notice before the oral hearing date. If the Requester fails to participate without proper reason or permission, the Request will be deemed to be withdrawn. The final CNIPA decision will state whether the generic drug infringes the patent and provide reasons. The judgment will be sent to the NMPA and published (with confidential information redacted).
Confidentiality
As part of this hearing, the generic drug applicant will have to provide specific information about its drug product, which may involve confidential information. Any confidential information obtained during the process shall be kept confidential.
The timelines for these procedures is quite short, and therefore adjudication may be quick.
Concluding Thoughts
In general, these rules are reasonably consistent with other international versions of patent linkage. The Chinese version has its own unique twists, such as the shorter and faster timelines, exceptions for traditional Chinese medicine, and a longer exclusivity for the first generic to the market. All in all, this linkage is a welcomed addition to the larger Chinese legal landscape, which has been developing at breakneck speeds in the past five years.
We are relieved that this final version of the rules adds a notice requirement to the MA holder/patentee regarding the generic drug applicant’s certification, which makes the 45-day window (to take action against the generic) at least more palatable. Unfortunately the 9-month stay still only applies to chemical inventions, and is not as long of a period as we would like. Nevertheless, the introduction of patent linkage into Chinese law is certainly exciting news for the biopharmaceutical industry worldwide.
These short timelines make it all the more important for foreign companies to engage proper Chinese counsel early in order to be completely prepared to respond when the generic companies come “knocking”.
Related Articles
How will Civil Cases Work under the Patent Linkage Provision in the New Chinese Patent Law?
CNIPA Releases Interim Measures for the Amended Chinese Patent Law Going Into Effect June 1, 2021A Detailed Dive into China’s New Patent Term Extension Provisions
-
In China, these are written in Swiss style format (Use of a composition for the manufacture of a medicament for …”) ↑
-
If the marketing authorization holder is not the patentee, then it needs to notify the patentee ↑
-
The generic drug applicant can only file a Request if the marketing authorization holder did not initiate a lawsuit or administrative proceeding within the 45-day window. Similarly, exclusive licensees can only file a Request if the patentee did not file a Request. ↑
About the Authors

Jennifer Che, J.D. is Vice President and Principal at Eagle IP, a Boutique Patent Firm with offices in Hong Kong, Shenzhen, and Macau.

Yolanda Wang is a Principal, Chinese Patent Attorney, and Chinese Patent Litigator at Eagle IP, a Boutique Patent Firm with offices in Hong Kong, Shenzhen, and Macau.
This article is for general informational purposes only and should not be considered legal advice or a legal opinion on a specific set of facts.





