Precision medicine is rapidly transforming the global healthcare landscape, providing more effective therapies and better patient outcomes through targeted solutions. As therapeutics move away from the traditional “one-size-fits-all” model, unique challenges are presented in the patent examination process. The 2024 Top 10 Patent Re-examination and Invalidation case highlighted below provides further insight into how the China National Intellectual Property Administration (CNIPA) interprets claim scope and inventive step during the examination of precision medicine technologies. Background Generic Drug maker Chia Tai Tianqing Pharmaceutical Group filed an invalidation request before the Patent Re-examination Board (the “Board”) against an invention patent titled “Use of Degarelix1 in the Preparation of a Medicament for Treating…
- China, China Patent Office, CNIPA, Invalidation, Inventiveness, Patent, Patent Re-examination and Invalidation Department
-
What Microsoft’s Recent Patent Invalidation Case in China Teaches Us About User Interface Patents
Each year the CNIPA publishes its Top Ten Patent Re-examination and Invalidation Cases for the previous year. These cases are meant to be guiding cases, showcasing exemplary real-world decisions that clarify certain aspects of the law. Today we’ll be sharing about one of the Top 10 Invalidation Cases in 2024 involving Microsoft (China) Co., Ltd. petitioning to invalidate a patent by Newman Infinite Inc. in the field of human-computer interaction (HCI). Case summary The patent claimed methods and devices for manipulating a touch screen user interface that could help differentiate between various touch targets, preventing undesired interactions with non-target elements. The patentee used a self-defined term “clutch user interface element”,…
-
Beijing Supreme People’s Court Upholds Novo Nordisk Semaglutide Patent in China based on Post-Filing Data
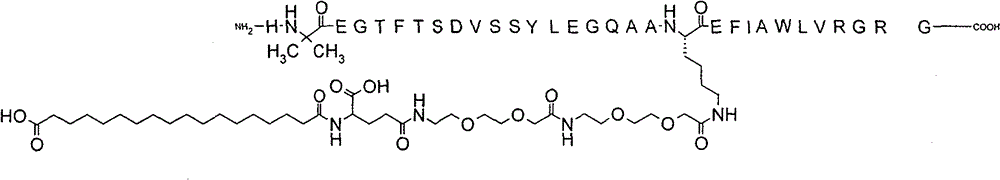
On December 31, 2025, The Supreme People’s Court upheld the Beijing IP Court’s decision, meaning that the semaglutide patent remains valid and will expire on March 20, 2026. Novo Nordisk announced this positive news via a press release on the day the case was decided. The Chinese Ministry of Commerce has also confirmed this decision on their website. This case is certainly positive news for those in the biopharmaceutical industry thinking of developing their drugs in China. Additionally, if you’ve been following this case, you’ll know that the validity of the patent came down to whether the Court would accept post-filing data demonstrating semaglutide’s surprising pharmacokinetic effects in animal models.…