Post-filing data in China has been a constant issue for many patent practitioners around the world. Examiners seem to require it often, and yet the rules regarding when it is acceptable have seemed much stricter than other jurisdictions worldwide. In fact, we tried to summarize the latest state of the law in an earlier blog post The Latest on Post-Filing Data in China’s Patent Law back in April 2020.
A lot has happened around the world this past year (to say the least). In the area of post-filing supplemental data, several different moving parts have contributed to more clarity in China on this issue.
First, Phase 1 of the US and China Economic and Trade Agreement signed January 15, 2020 included specific provisions where China “shall permit pharmaceutical patent applicants to rely on supplemental data to satisfy relevant requirements for patentability, including sufficiency of disclosure and inventive step . . .” (Article 1.10)
Exactly one year later China made good on that promise, publishing new Examination Guidelines that not only explicitly directed patent examiners to consider post-filing supplemental data when considering inventive step and sufficiency, but also provided a number of examples. Although the trade agreement was part of the impetus, the Guidelines indicate that they were responding to requests from both domestic and foreign innovators.
We will explore the Examination Guidelines in a 3-part series. Today’s Part I will cover the latest change regarding post-filing supplemental data. Part II will explore examples on novelty and inventive step for chemical compounds. Part III will explore examples on inventive step for biological inventions.
Sufficiency and Inventive Step
The newest Examination Guidelines clearly state that examiners shall consider post-filing supplemental data when considering both inventive step and sufficiency if the supplemental data could undoubtedly be deduced by a skilled person in the art from the disclosure as originally filed.
The Guidelines provide two very useful examples where post-filing data is acceptable.
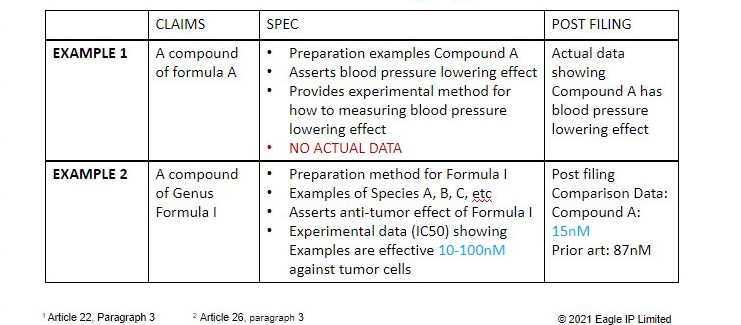
Example 1
Example 1 illustrates a situation where post-filing efficacy data for a compound can be considered. In Example 1, the claims are directed towards a compound of Formula A. The specification includes examples teaching how to make the compounds of formula A, and asserts that these compounds have the biological effect of lowering blood pressure. The specification also provides methods teaching how to measure the blood pressure lowering effect of the compounds, thus teaching how to test their efficacy. However, the specification contains no actual efficacy data.
The Examiner must consider post-filing data that confirms the efficacy of the compounds in their ability to lower blood pressure, provided that one of skill in the art, based on all that had been provided, would undoubtedly deduce such efficacy based on the original disclosure. In this case, one of skill in the art would believe such data because the original application taught that the claimed compounds had such biological effect.
Do keep in mind that the Examiner is only required to consider the post-filing data, and may still choose to reject a claim based on a number of other reasons.
Example 2
Example 2 demonstrates a situation when post-filing data that technically provides new information (not specifically disclosed in the original disclosure) is still acceptable. In short, Examiner shall consider supplemental data that was presented to overcome prior art that was not previously known.
In Example 2, the claims are directed towards a genus of compounds of Formula I. The specification includes processes for preparing the compounds of Formula I, a number of species (A, B, C, etc.), and an assertion that the compounds of Formula I have an anti-tumor effect. This specification does provide actual data, but only discloses that the compounds are efficacious against tumor cells at a concentration range of 10 – 100nM. No exact data is provided.
The Examiner later rejects the claims based on a prior art reference showing that a close compound has the asserted efficacy at a concentration of 87nM, which falls within the range of the claimed compounds.
The Applicant can submit post-filing data showing that a compound of formula A actually has a surprising technical effect of 15nM efficacy against tumor cells. The Examiner must consider this data. Provided that the specification sufficiently discloses and has support for compound A, applicant should be able to amend the claims towards Compound A in order to overcome inventive step.
Please note that although “new data” can be presented to overcome an inventive step rejection, all claim amendments still must have support in the specification as originally filed. Accordingly, this makes it all the more important that if an applicant wants to rely on supplemental data during prosecution, the applicant should ensure that the application as originally drafted includes multiple back-up scopes in the specification to provide maximum flexibility in claim amendments down the road.
Initial Thoughts
These supplemental data examples are quite encouraging in that they provide two very real situations that life science applicants face all the time. Research and development is an ongoing process, and pharmaceutical and biotech companies are always balancing the tension between earlier filing dates and sufficiency of data generated by that time. These examples at least give us some hope that more post-filing data can be considered.
At the same time, we do note that the standard of “undoubtedly deduce” is still quite high, and it will take time for us to understand where that line actually exists in the biotech and chemical arts.
All Posts in This Series: China’s Newest Examination Guidelines
Part I: Post-Filing Supplemental Data for Compounds
Part II: Novelty and Inventive Step for Compounds
Part III: Inventive Step for Biological / Life Science Inventions
Official Updated Examination Guidelines (Chinese only)
This article is for general informational purposes only and should not be considered legal advice or a legal opinion on a specific set of facts.
About the Authors

Jennifer Che, J.D. is Vice President and Principal at Eagle IP, a Boutique Patent Firm with offices in Hong Kong, Shenzhen, and Macau.

Yolanda Wang is a Principal, Chinese Patent Attorney, and Chinese Patent Litigator at Eagle IP, a Boutique Patent Firm with offices in Hong Kong, Shenzhen, and Macau.

Sally Yu is a Chinese Patent Attorney at Eagle IP, a Boutique Patent Firm with offices in Hong Kong, Shenzhen, and Macau.





