Update! The finalized Implementation Measures are now out. You can read the details here: Breaking: China Released New Implementation Measures for the New Patent Linkage System Last month September 11, 2020, China’s National Medical Products Administration (NMPA) and the China National Intellectual Property Administration (CNIPA) jointly issued a draft set of measures for public opinion about early dispute resolution mechanisms for drug patents, what is referred to as a patent linkage system. Below is a summary highlighting key provisions. Creation of an “Orange Book” List The NMPA will create a public registration platform that will list patent information for all drugs marketed or seeking marketing authority in China (similar to…
-
-
Polymorph Patents in China: What is the Standard for Inventiveness for New Crystal Forms?
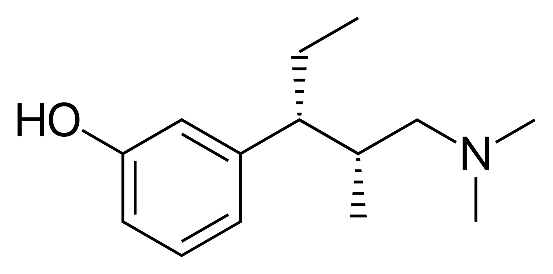
This case is focused on polymorphs, namely what are the standards for novelty and inventiveness when it comes to new crystal forms of a known drug? A recent Supreme People’s Court decision in China is illustrative of the way Chinese courts are thinking about polymorph patents in China. Grünenthal is a German pharmaceutical company and inventor of the opioid painkiller Pentadol[1]. Grünenthal has a Chinese patent (ZL 200580021661.1) with claims directed towards a new crystalline form of particular phenol hydrochloride (“tapentastat hydrochloride” and “Form A” in claim 1), processes for preparing tapentastat hydrochloride (claim 3), and processes for preparing Form A (claims 16 and 23). Form A is made by…
- China, Patent Linkage, Patent Term Extension, Pharma, Proposed Changes, Regulatory, Updates and Changes
Breaking News: Newest Draft Amendment to the Chinese Patent Law Available for Public Comment
Finally, after more than a year since the last draft amendment came out in 2019, a new draft has been submitted to the National People’s Congress Standing Committee for deliberation on June 28, 2020. The newest version is now published for public comments until August 16, 2020. As a whole, these proposed changes address a lot of the criticisms people have had regarding the strength of IP protection in China. These changes, once implemented, will make China a much more patent-friendly jurisdiction, benefiting innovators worldwide. Examples of notable changes include (1) a longer patent term for new drugs and design patents; (2) patent linkage; (3) litigation reform such as significantly…