This case is focused on polymorphs, namely what are the standards for novelty and inventiveness when it comes to new crystal forms of a known drug? A recent Supreme People’s Court decision in China is illustrative of the way Chinese courts are thinking about polymorph patents in China.
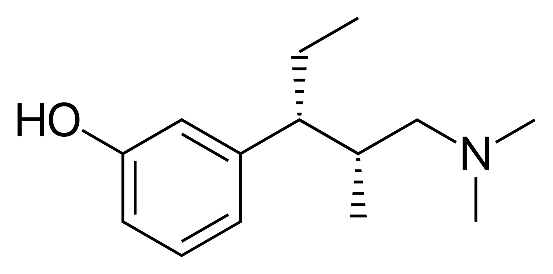
Grünenthal is a German pharmaceutical company and inventor of the opioid painkiller Pentadol[1]. Grünenthal has a Chinese patent (ZL 200580021661.1) with claims directed towards a new crystalline form of particular phenol hydrochloride (“tapentastat hydrochloride” and “Form A” in claim 1), processes for preparing tapentastat hydrochloride (claim 3), and processes for preparing Form A (claims 16 and 23). Form A is made by dissolving Form B in acetone, acetonitrile or isopropanol, and crystallizing out Form A.
The Invalidation
On June 23, 2015, Jiangsu Hengrui Pharmaceutical Co., Ltd. (“Henrui”) filed an invalidation request against Grünenthal’s polymorph patent with the Patent Re-examination Board of the State Intellectual Property Office (“PRB”) requesting that all patent claims be declared invalid in view of five prior art references. Henrui argued that the all claims were not inventive in view of the references. Three of the references were used by the PRB (and later the courts) to evaluate the patentability.
- Prior Art 1: A 1996 Chinese patent publication disclosed the same compound as being converted to a salt and then crystallized. The specification did not provide sufficient details regarding the crystallization method, but merely said that it was crystallized from a free base. The disclosure also said that compounds of formula 1 “can be converted to its salt with an acid known in the art, such as hydrochloric acid.” It included a list of commonly used crystallization solvents for the formation of salts, including diethyl ether, diisoethyl ether, alkyl acetate, acetone and 2-butanone.
- Prior Art 3: A journal article[2] summarizing drug polymorphism research taught that most organic drugs exist in polymorphic forms, and different crystalline forms can be obtained under different recrystallization conditions and ambient temperatures. There is a lattice energy difference between the polymorphic forms of the drug. The low-energy crystal form is called the stable form, and the high-energy form is called the meta-stable form.
- Prior Art 4: An organic chemistry textbook[3] taught that recrystallization is one of the common methods for purifying solid organic compounds, and taught how to select recrystallization solvents as well as how to do a crystallization. Notably, it mentioned that acetone and acetonitrile are commonly used solvents for recrystallization.
Post-Filing Unexpected Data
Grünenthal responded to the allegations of obviousness by submitting post-filing experimental data showing that Form A had unexpected technical properties, namely that it was more stable under pressure at ambient temperature. Grünenthal subjected both Form A and Form B to a pressure of 2 tons for 60 seconds. All batches of Form B had changed but Form A was the same, and thus surprisingly more stable.
The PRB decided that all the claims were obviousness in view of the combination of prior art references 1, 3, and 4. As a result, the patent was declared invalid.
Administrative Litigation
Grünenthal appealed and argued that the claimed invention was inventive because a crystal’s specific form is unpredictable (many factors, such as solvent, temperature, cooling time, affect a crystal form); and the cited art did not teach or suggest (1) the specific details of Form A; nor (2) Form A’s unexpected stability under ambient conditions.
Was Unexpected Improved Stability Already Established in the Specification As Filed?
Grünenthal argued that SIPO’s interpretation of the technical problem was wrong. It was not to provide alternate molecules with different crystal forms, but to provide a crystal form with improved stability. Grünenthal pointed to specific paragraphs in the specification[4] showing that the idea of stability was already established. Accordingly, the post-filing data presented in court was not a new effect.
The other two parties disagreed, arguing that the patent only asserted the effect without providing any data to prove the unexpected technical effect. Although Grünenthal pointed to specific examples in the specification, those examples[5] only explained that at certain temperatures (e.g., at ‑40° C and 40°C to 50° C), Form A and Form B can converted between each other, but said nothing about either one’s stability at ambient temperature. It was unclear when a conversion would occur at temperatures from ‑40 °C to 40 °C. None of the data showed that at ambient temperature, form B was unstable and would transform into crystalline form A. Without unexpected data, structurally close known compounds cannot have inventive step.
Furthermore, they argued that the patent was not enabled for the specific crystal form because it only provided general teachings for some common crystallization methods, not the ones that would lead to the new crystal form with improved stability. Finally, even if it were enabled, the crystal form was not inventive because one of skill in the art would be motivated by the prior art to select a common used solvent (e.g., acetone) to crystalize the compound at room temperature.
The Beijing IP Court’s Analysis
According to Article 22, Paragraph 3 of the Patent Law, inventiveness means that the invention has outstanding substantive features and significant progress compared with the technology existing before the filing date.
Claim 1 – Distinguishing feature: only the crystal form
The Court compared the crystal form A of claim 1 with the closest prior art and determined that the only difference was the crystal form, as exhibited by Form A’s unique X-ray pattern. Accordingly, they defined the problem to be solved to be: providing alternative crystal forms of tapentastat hydrochloride
The Court agreed with Grünenthal that the patent specification asserts Form A is more stable than Form B under external environmental conditions. However, the data was insufficient, only showing that Form A transforms to Form B between 40 ° C to 50 ° C; and Form B transforms to Form A at -40 ° C when stored for 72 hours. Therefore, one of skill in the art could not determine the transformation between Form A and Form B within the temperature range of -40°C to 40°C, and thus could not determine which form was more stable at ambient temperature.
Additionally, Grünenthal’s stress test data only showed the stability of Forms A and B under different pressure conditions, which was never taught in the original specification. Therefore, the post-filing data could not be accepted and used to prove the claimed effect (even if it may have been “surprising” enough to demonstrate inventiveness).
Finally, the Court indicated it could not verify whether the stress test sample was the same as Form A in the patent application. The Court concluded Grünenthal could not prove the stability of Form A.
Accordingly, the Court judged that the technical problem actually solved was providing a polymorph of tapentastat hydrochloride in a different crystalline form to meet the needs of drug production and use, and thus could be done through routine experimentation.
The Court further emphasized that where there are multiple crystalline forms for the same compound, a newly developed crystalline form is not, by default, inventive. This is because one skilled in the art generally has motivation to improve the existing crystalline form, and in this case, the solution itself was based on known methods and was not itself technically challenging. In this case, only if the new crystalline form achieves unexpected technical effects relative to the existing technology, can it be considered inventive. Claim 1 is not inventive because there is no evidence that Form A has achieved any unexpected technical effects relative to the prior art.
Claims 3-15: Method of Making Crystal Form A
Claims 3-15 relate to the method of making crystal form A. Because the closest prior art is a different polymorph, the crystallization methods involve different starting materials and different solvents.[6] However, the Court was of view that there was no obvious difference in the technical effects because the choice of starting materials had little effect on the crystal form obtained by crystallization. For example, the hydrochloride solution obtained after dissolution of the starting materials was the same and resulted in no obvious difference in the technical effects (providing crystal form A). Accordingly, the claimed method was obvious in view of the prior art, which taught that organic drugs exist in polymorphic forms which can transition from one form to another via recrystallization in commonly known solvents, such as acetone.
The Court concluded that claim 3 did not have outstanding substantive features and significant progress in view of the prior art, and thus did not possess inventiveness as spelled out in Article 22, paragraph 3 of the Patent Law.
Claims 4-15, which further describe crystallization temperature, seed crystal and specific crystallization operations, were considered standard and not inventive.
The Beijing IP Court affirmed the Patent Reexamination Board’s invalidation of the patent.
Grünenthal appealed to the Supreme People’s Court.
The SPC Decision – Sep 29, 2019
The Supreme People’s Court affirmed all facts found in the first trial and only focuses on two issues: whether claim 1 and claim 3 were inventive.
The key question to ask is “what is the technical problem that was solved?” To answer this question, one must evaluate the technical effect that the distinguishing features can actually achieve in the patent.
The Court identified the technical effect as Form A’s improved stability over Form B. The next step would be to evaluate whether one of skill in the art would conclude that Form A had improved stability over Form B based on the contents of the specification. The Court indicated that in the field of medicine (an unpredictable art area), experimental proof is required to establish a claimed effect. Accordingly, the Court concluded the specification did not have sufficient evidence of Form A’s stability over Form B. Grünenthal’s stress test was not sufficient because one of skill in the art would not conclude that particular technical effect (Form A’s improved stability under pressure) at the time of the filing date.
In summary, the Court decided that the technical problem actually solved by Grünenthal was to provide alternative crystal forms of tapentastat hydrochloride. The solution is well known in the art, routine, and lacking surprising technical effect, is also not inventive.
Similarly, claim 3 (the method) was also upheld as invalid because the method of recrystallization using known solvents was not particularly inventive.
The appeal was rejected and the original sentence was upheld and made final.
Polymorph Patents in China: Inventive Step Requires Sufficient Surprising Technical Effects
In many ways this decision is not surprising. It shows once again the importance of demonstrating clearly and sufficiently proving an invention’s surprising technical effects in the specification. In this case, even though the specification asserted that Form A was more stable than Form B, the data itself was insufficient to convince one of skill in the art that Form A’s surprising aspect over Form B (namely, increased stability under pressure at ambient temperature), was true at the time of filing. Post-filing data could not cure the problem in this case because the original idea was not established sufficiently.
Once again, this decision reiterates the importance of including as much data as possible to support different surprising technical aspects of an invention. Just asserting certain ideas (and backing it up later with post-filing data) is not sufficient. How much data is enough? It will vary depending on the nature of the technology, the state of the prior art, and the level of the skilled practitioner. Minimally, the data must be sufficient to convince one of skill in the art that the invention had the particular effect at the time of filing.
About the Authors

Jennifer Che, J.D. is Vice President and Principal at Eagle IP, a Boutique Patent Firm with offices in Hong Kong, Shenzhen, and Macau.

Yolanda Wang is a Principal, Chinese Patent Attorney, and Chinese Patent Litigator at Eagle IP, a Boutique Patent Firm with offices in Hong Kong, Shenzhen, and Macau.
APPENDIX
Claims
1. (-)-(1R, 2R) -3- (3-dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride crystal form A, when measured using CuKα radiation The data of the X-ray pattern of Form A is basically shown in the following table:
|
Peak number |
l |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
|
°2θ |
9.07 |
10.11 |
14.51 |
15.08 |
15.39 |
15.69 |
15.96 |
16.62 |
17.00 |
|
Peak number |
l0 |
ll |
l2 |
l3 |
l4 |
l5 |
l6 |
l7 |
l8 |
|
°2θ |
18.24 |
18.88 |
20.00 |
20.39 |
21.66 |
22.54 |
24.27 |
25.03 |
25.47 |
|
Peak number |
l9 |
20 |
2l |
22 |
23 |
24 |
25 |
26 |
27 |
|
°2θ |
25.84 |
26.04 |
26.94 |
27.29 |
27.63 |
28.33 |
28.72 |
29.09 |
29.29 |
|
Peak number |
28 |
29 |
30 |
31 |
32 | ||||
|
°2θ |
29.76 |
30.37 |
30.74 |
31.70 |
34.37 |
3. A method for preparing (-)-(1R, 2R) -3- (3-dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride in Form A, by combining Form B (-)-(1R, 2R) -3- (3-dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride dissolved in acetone, acetonitrile or isopropanol, so that the solution crystallizes and separates crystals of (-)-(1R, 2R) -3- (3-dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride in Form A.
23. Method for preparing crystal form A of (-)-(1R, 2R) -3- (3-dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride, comprising the steps of: dissolving (-)-(1R, 2R) -3- (3-dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride of Form B together with activated carbon in acetonitrile, heating the solution to the boiling point, removing the activated carbon by filtration, stirring the solution at a temperature below 40 ° C, removing the insoluble residue by filtration and removing part of the solvent to make (-)-(1R, 2R) of Form A -3- (3-Dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride crystals, then dissolving the crystals obtained in acetonitrile, removing the insoluble residues and parts by filtration, crystallizing (-)-(1R, 2R) -3- (3-dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride of Form A. “
Footnotes
- Pentadol is (-)-(1R, 2R) -3- (3-dimethylamino-1-ethyl-2-methyl Propyl) –phenol ↑
- Research progress of drug polymorphism”, Shen Jianlin et al., “Chinese Journal of Hospital Pharmacy”, 2001, Vol. 21, No. 5, pp. 304-305 Paragraph 2, the last paragraph in the right column, the fifth paragraph in the left column on page 305). ↑
- “Organic Chemistry Experiment” (2nd Edition), edited by the Institute of Organic Chemistry, School of Chemistry, Peking University, Guan Ye, first revision, Peking University Press, 2002, pages 39-46, section 3.1 (see particularly Table 3.1 on page 40). ↑
- Paragraph [0005] in the specification said that Form A “is very stable in the surrounding environment and can therefore be used to prepare pharmaceutical compositions.” Paragraph [0029] states: “Form A according to the present invention has the same pharmaceutical activity as Form B, but it is more stable under external environmental conditions.” ↑
- Paragraphs [0056] to [0057] stated:” Example 5: Preparation of Form A (4). Preparation according to Example 25 of European Patent EP693475B1 ( -)-(1R, 2R) -3- (3-dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride. The (-)-(1R, 2R) thus synthesized -3- (3-Dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride was stored at -40 ° C for 72h. (-)-(1R, 2R) -3- (3 -Crystalline form A of dimethylamino-1-ethyl-2-methylpropyl) -phenol hydrochloride, which is confirmed by X-ray powder diffraction and Raman microscopic analysis. Paragraphs [0897] to [0898] Paragraph stated: “Example 16: Variable temperature X-ray powder diffraction experiment. A variable temperature X-ray powder diffraction experiment was performed, thereby generating crystal form B from crystal form A. During this experiment, from 40 to 50 ° C, Form A transforms to Form B. The result is reversible, and at lower temperatures, Form B transforms to Form A.” ↑
- Claim 3 is to dissolve the crystal Form B in acetone and acetonitrile or isopropanol, and then crystallize Form A from the solution. Prior art document 1 is to dissolve the free base in 2-butanone, add trimethylchlorosilane / water to convert the free base into the hydrochloride, and then crystallize out crystals. ↑




2 Comments
eagleip
Thank you!
Zuber Deshmukh
HI,
Thanks for the updates, love to read and update IP development in China.
Regards
Zuber