Crystalline forms are critical to pharmaceutical patents, offering extended protection for improved stability, bioavailability, or manufacturability. However, securing such patents in China has grown increasingly difficult due to the China National Intellectual Property Administration (CNIPA)’s strict patentability criteria. Unlike the U.S. or Europe, where structural novelty or problem-solving utility may suffice, China demands quantifiable evidence of superiority over prior art forms and rejects patents based on routine screening alone. Recent decisions, like the invalidation of fruquintinib Crystal Form I, highlight common pitfalls: insufficient comparative data, incremental technical effects, and failures to preempt obviousness challenges. With China’s pharmaceutical market surging and secondary patents under heightened scrutiny, companies must strategically align their IP strategies…
- China, China Patent Office, CNIPA, data, Invalidation, Patent, Patent Law, Patent Re-examination Board, Patentability, Pharma, Support Requirements
-
RNAi Patent Success in China: Overcoming “Comprising” Claim Challenges
An Update on Sufficiency and Inventiveness of RNAi Patents in China RNAi is a fast-developing technology that has gained traction in the pharmaceutical industry as a promising therapeutic agent. It is important to follow closely RNAi patent proceedings to learn how different examination boards and courts understand and handle these new technologies. The first-ever invalidation decision in China for an RNAi patent1 was rendered in 2022 by the Patent Re-examination Board (the “Board”). We previously wrote about this case, which discussed the standards for post-filing data, sufficiency, and inventive step, particularly for RNAi inventions. Since then, two newer cases involving RNAi have also been upheld by the Board after facing similar…
-
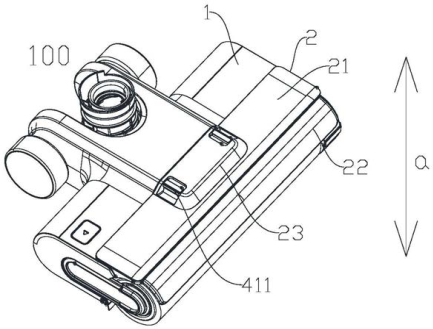
How a Secondhand Ground Brush Wiped Away a Chinese Patent
A Chinese robot vacuum company digs up “hidden evidence” to successfully invalidate a patent owned by its biggest competitor. Highlights Background With the growth of online shopping, it is easier than ever to provide evidence of a sale when the product is still being sold on the market. But what if the product is no longer being sold? It’s much more challenging to prove the original disclosure date for a secondhand item, especially in a jurisdiction like China, which has very stringent notarization and legalization1 requirements for introducing evidence into a proceeding. The case below demonstrates an example of how one major Chinese robot company successfully dug up and introduced…