The Supreme People’s Court has just issued draft regulations for comment regarding how new Article 76 will work in conjunction with civil procedure law. The period to submit comments ends December 14, 2020, and the final version will come into force June 1, 2021, together with the new Chinese Patent Law. The Backdrop: New Chinese Patent Law Adds Patent Linkage (Article 76) Linking Drug Approval to a “Clear” Patent Position In October of 2020 China passed the 4th amendment of the patent law which ushered in some sweeping changes in certain areas that are especially exciting for life science companies. One of the most important changes is the introduction of…
- Biotech, China, Patent Linkage, Patent Term Extension, Pharma, Supreme People's Court, Updates and Changes
-
Polymorph Patents in China: What is the Standard for Inventiveness for New Crystal Forms?
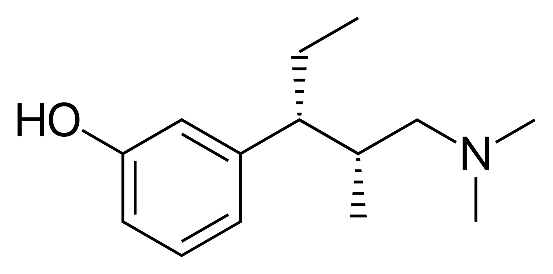
This case is focused on polymorphs, namely what are the standards for novelty and inventiveness when it comes to new crystal forms of a known drug? A recent Supreme People’s Court decision in China is illustrative of the way Chinese courts are thinking about polymorph patents in China. Grünenthal is a German pharmaceutical company and inventor of the opioid painkiller Pentadol[1]. Grünenthal has a Chinese patent (ZL 200580021661.1) with claims directed towards a new crystalline form of particular phenol hydrochloride (“tapentastat hydrochloride” and “Form A” in claim 1), processes for preparing tapentastat hydrochloride (claim 3), and processes for preparing Form A (claims 16 and 23). Form A is made by…