What is the standard of sufficient disclosure for AI-related patents: A case study in China The rapid growth of AI patent applications present significant challenges to existing patent application and examination practices. One of them is satisfying the requirement for sufficient disclosure. The following case was featured in the 2023 CNIPA Compilation of Key Decisions on Patent Reexamination and Invalidation Cases (“2023 Compilation”) as an example to elucidate the current standards for sufficient disclosure in examining AI patent applications in China. Rejection of Alipay’s AI + Brainwave Patent The applicant Alipay1 had a Chinese Patent2 on an invention relating to a method and system that trains and utilizes AI models…
-
-
How a Secondhand Ground Brush Wiped Away a Chinese Patent
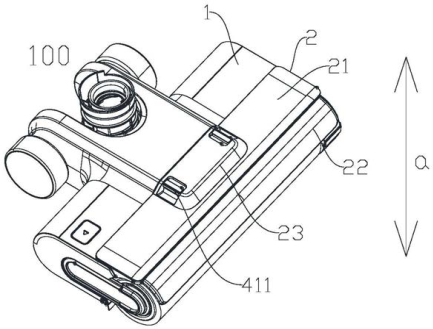
A Chinese robot vacuum company digs up “hidden evidence” to successfully invalidate a patent owned by its biggest competitor. Highlights Background With the growth of online shopping, it is easier than ever to provide evidence of a sale when the product is still being sold on the market. But what if the product is no longer being sold? It’s much more challenging to prove the original disclosure date for a secondhand item, especially in a jurisdiction like China, which has very stringent notarization and legalization1 requirements for introducing evidence into a proceeding. The case below demonstrates an example of how one major Chinese robot company successfully dug up and introduced…
-
New Fee Standards Released for Chinese Patent Applications
Recently, the Chinese government issued four official notices introducing new fee standards and deduction rules for Chinese patent applications.1 These updates address international applications (PCT applications) and the Chinese national phase applications thereof, as well as patent term adjustment/extension (PTA/PTE). Please find our summary below: PCT application fee deduction rules Application fee & extra fees (claims/pages) Substantive examination fee PCT application type Before Jul 26, 20242 After Jul 26, 2024 Before Jul 26, 2024 After Jul 26, 2024 CNIPAas RO CNIPA as ISA or IPEA 100% OFF 100% OFF 100% OFF 100% OFF non-CN as ISA or IPEA EPO as ISA JPO as ISA SPO as ISA 100% OFF…