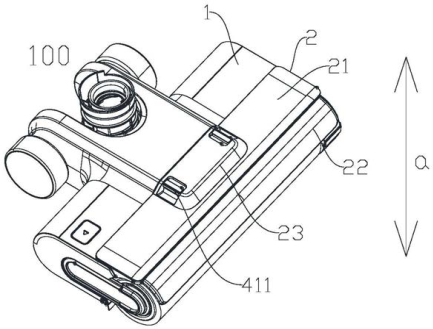
A Chinese robot vacuum company digs up “hidden evidence” to successfully invalidate a patent owned by its biggest competitor. Highlights Background With the growth of online shopping, it is easier than ever to provide evidence of a sale when the product is still being sold on the market. But what if the product is no longer being sold? It’s much more challenging to prove the original disclosure date for a secondhand item, especially in a jurisdiction like China, which has very stringent notarization and legalization1 requirements for introducing evidence into a proceeding. The case below demonstrates an example of how one major Chinese robot company successfully dug up and introduced…
-
-
Beijing IP Court Reverses CNIPA Decision and Upholds Ozempic® semaglutide patent in China as VALID based on Novo Nordisk’s Post Filing Data
Recently, all eyes have been on China as the fundamental patent covering semaglutide, the active ingredient in Ozempic® and Wegovy®, will expire on March 20, 2026. It goes without saying that generics are ramping up bigtime in China (and also around the world), preparing to manufacture and sell this blockbuster drug to one of the biggest markets in the world. Any shortening of the patent term for this key semaglutide patent in China could cause an immediately shift in the Chinese Ozempic market (not to mention directly impacting Novo Nordisk). Novo Nordisk’s Semaglutide Patent in China On September 5, 2022, the China National Intellectual Property Association (China’s patent administrative office,…
-
Twice the Trouble: Unraveling a Single Case of Dual Patent and Trademark Infringement
Can a rights holder sue the same infringer separately based on one single infringing act that infringes both trademark and patent rights? An interesting case this year from China’s Supreme People’s Court that addresses this specific issue ((2023)最高法知民终235号). Beijing Run De Hong Tu Technology Development Co., Ltd. (“Run De Hong Tu”) sued an individual named Li XX[1] for patent infringement based on his activities as a small retailer selling a certain sewage pipe branded as “Submarine”. In fact, Run De Hong Tu had initiated multiple similar lawsuits, primarily targeting small and micro retailers, and had been able to secure substantial compensation in these cases. Run De Hong Tu, along with…