Post-filing data can sometimes be the crucial difference between a patent allowance and a final rejection. The reasons are endless why important data may not have been included in the original patent application as filed. Time and budget may have been insufficient to generate data over the full scope of the claims at the time of filing. The priority of a company’s business may have changed, meaning now more resources are available to generate data in this new area. Whatever the reason, patent practitioners often rely on data generated after the filing of the original application in order to overcome rejections from the patent office. Traditionally, China has been known…
-
-
China Provides Specific Directions to Strengthen Patent / Technology Protection from 2020 to 2021
China continues to progress towards its major goal of significantly strengthening IP protection within its borders. Last year it announced several proposed amendments to its patent laws. In January 2020 China and the US signed the Economic and Trade Agreement between the Government of China and US (published 16 January 2020 –“Trade Agreement”), which detailed several areas in which China agreed to reform its laws, including in the areas of trade secrets and confidential information, pharmaceutical-related IP, piracy and counterfeiting, and enforcement. Just a few days ago on April 20th, 2020, the Chinese National Intellectual Property Administration (CNIPA) published a long and comprehensive plan (“Plan”) on how to strengthen the…
-
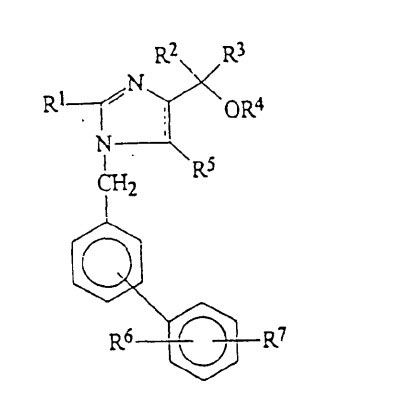
Markush claims in China – what can be arbitrarily deleted during invalidation?
Since 2010, the China Patent Re-examination Board (PRB) has published the top 10 patent invalidation cases of the year in April of each year. The selection criteria are high social concern, significant impact on the related industry, or involve difficult legal issues and important examination criteria. Below is one of the top 10 cases that discusses post filing data in China patents. This case (Beijing Winsunny Harmony Science & Technology Co., Ltd. v. Daiichi Sankyo Co., Ltd) describes an invalidation request of Daiichi Sankyo’s Chinese invention patent 97126347.7 related to processes of preparing pharmaceutical compositions for treating or preventing hypertension. The patent covered the marketed hypertension drug Olmesartan medoxomil. During…