Although PTE has been in the Chinese Patent Law since the 4th Amendment of the law came into effect June 1, 2021, we didn’t really know the exact details of how it operated until the Implementation Regulations and Examination Guidelines (“Implementation Regulations”) were finally released in December 2023.
New Rules 77-84 in the Implementation Regulations discuss patent term compensation due to regulatory delay (“PTE”). In this article, we consolidate our summary about PTE based on these latest updates.
Highlights
- Eligibility for PTE: products, processes of preparation, and medical use patents directed to an innovative drug, or improved drug (including chemical, biological, or traditional Chinese medicine (TCM)) that hasn’t been marketed overseas
- PTE Term: similar to Europe
- Limitations of PTE: One patent per drug, one drug per patent, 5 years PTE, 14 years total patent term post drug approval
Patent Term Extension (PTE)
Article 42.3 of the Patent Law states that “[i]n order to compensate for the time taken for the review and approval process before the marketing of a new pharmaceutical product, the patent administration department under the State Council shall, at the request of the patentee, extend the term of the new pharmaceutical-related invention which has been approved for marketing in China. The compensation term may not be more than five years, and the total effective term of the patent right may not be more than fourteen years from the date of marketing approval.”
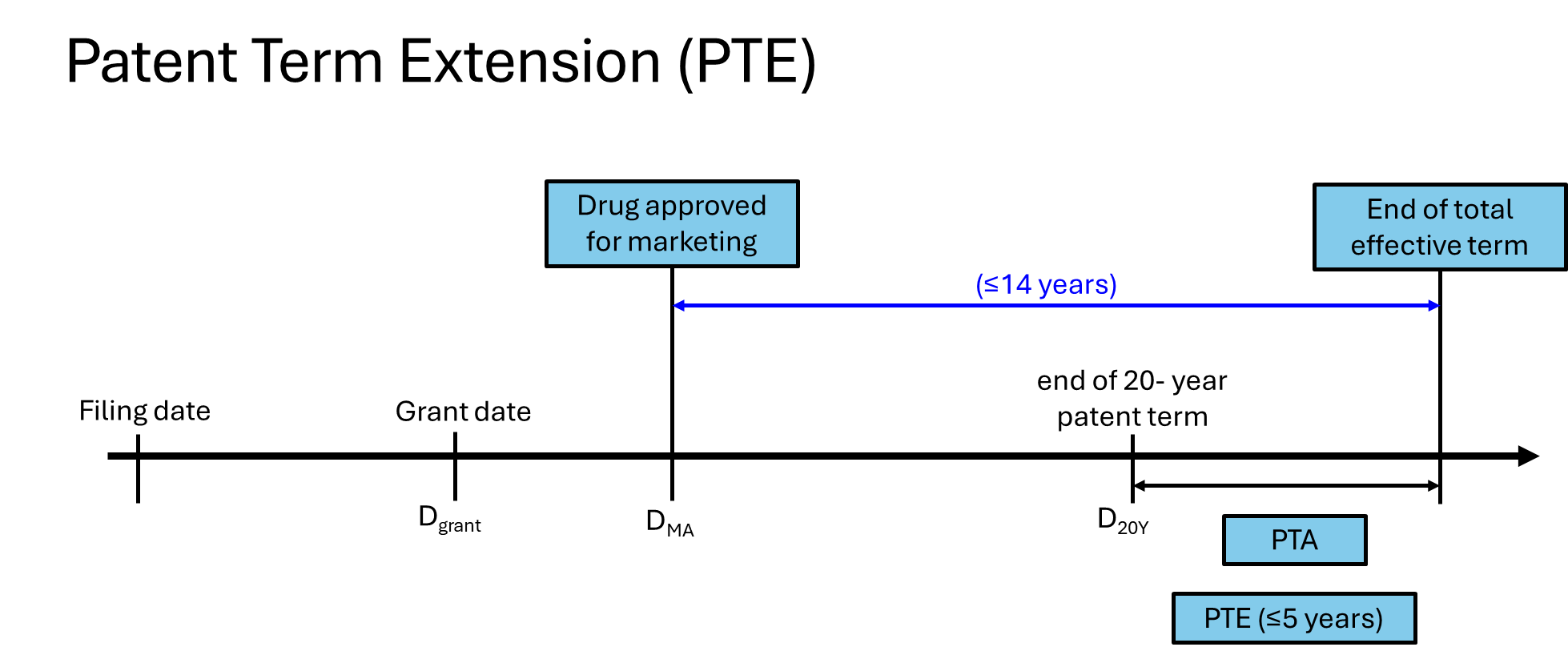
The CNIPA has provided some official1 equations showing the PTE calculation:
Equation 1: PTE compensation term = DMA – filing date – 5 years
Equation 2: Total effective term = (D20Y – DMA) + PTA compensation + PTE compensation term (≤14 years)
In the first equation, the PTE compensation term is limited to no more than 5 years, according to Article 42.3 of the Patent Law, where
DMA refers to the date when the drug is approved for marketing by the National Medical Products Administration (NMPA); and
The term of “minus 5 years” comes from Rule 82.2
In the second equation, the total effective term is limited to no more than 14 years after accounting for both the PTA and PTE, according to Article 42.3 of the Patent Law, where
D20Y refers to the end date of the 20-year patent term counted from the filing date of the granted application, and
DMA refers to the date when the drug is approved for marketing and marketing authorization is granted by the NMPA.
Note that the scope of PTE protection is more limited, only extending to the drug approved by NMPA and the relevant technical solution for the approved indications.
Furthermore, PTE is added on top of any PTA that the patent has, though limits still apply. Specifically, the patent office will only consider PTE after the request for PTA has been processed, unless the time limit for requesting PTA has already expired (3 months after grant of patent) or if the applicant has expressly abandoned their request for PTA. If the total effective term of the drug patent is already at 14 years or more (post approval), the request for PTE will be rejected.
Eligibility
Rule 80 in the Implementation Regulations indicates that drug product (composition of matter) patents, preparation method patents, and medical use patents covering a new drug is eligible for PTE.3 Notably, “new drug” refers to “innovative drugs” and certain “improved drugs” that have not been marketed overseas or domestically.
The terms “innovative drugs” and “improved drugs” come straight from the National Medical Products Administration (“NMPA”)4 classification system. Innovative drugs refer to certain types of medicinal products that have never been marketed worldwide (shown in Table 1), while the certain subcategories of improved drugs which have “obvious clinical advantage” refer to modifications or new uses of previously known active ingredients or drug products (see Table 2)
Table 1: Types of innovative drugs5
Innovative drugs | NMPA Category | Definition |
Chemical Drugs Category 1 | Innovative drugs that have not been marketed overseas or domestically, which contain compounds with new structures that are clearly defined, possess pharmaceutical effects and clinical value. | |
Preventive Biological Products 6(PBP) Category 1 | Innovative vaccines that have not been marketed overseas or domestically, including: | |
Therapeutic Biological Products 7(TBP) Category 1 | Innovative therapeutic biological products that have not been marketed overseas or domestically | |
Traditional Chinese Medicine (TCM) Category 1 | Innovative TCMs products that have not been registered in China nor marketed overseas, and possess clinical value |
Table 2: Subcategories of improved drugs eligible for PTE (referred to as ‘PTE Category’ below)8
PTE Category | NMPA Category | Improved drugs |
(1) | Chemical Drugs Category 2.1 | drugs arising from esters of known active ingredients or salts of known active ingredients |
(2) | Chemical Drugs Category 2.4 | drugs with new indications containing known active ingredients |
(3) | Preventive Biological Products Category 2.2 | vaccines improved from vaccine strains |
(4) | Therapeutic Biological Products Category 2.2 | biological products with new indications (which have not been approved domestically or oversea) |
(5) | TCM Category 2.3 | traditional Chinese medicine with added functions and indications |
Aside from PTE categories (1) – (5) above, other categories of improved drugs are not eligible for PTE, for example, new dosage forms containing known active ingredients in chemical drugs (Chemical Drugs Category 2.2) or new compound formulations containing known active ingredients (Chemical Drugs Category 2.3).
Scope of PTE Protection
During the PTE compensation period as described in Rule 83, the scope of protection of the patent shall be limited to the new drug approved for marketing by the NMPA, and the approved technical solutions related to the indications of the new drug.
According to Part 5 Ch.9 Section 3.5 of the Examination Guideline, the “technical solution” related to new drugs shall be based on the structure, formulation, and composition of the new drug approved by the NMPA, as well as the approved indications and production process recorded on file. If the designated claims do not include the technical solutions related to the new drug that has obtained marketing authorization, no PTE will be granted.
During the PTE compensation period, the protection scope of product claims is limited to the approved new drug product that is used for the approved indications. Likewise, the protection scope of medical use claims is limited to the approved indications of the approved new drug. The protection scope of preparation method claims is limited to the production processes recorded with the NMPA of the new drug for the approved indications. Within this scope of protection, the patentee’s rights and obligations are the same as before the PTE compensation period.
Requirements for Requesting PTA/PTE
Part 5 Ch.9 Sections 2 and 3 of the Examination Guidelines describes the requirements for requesting PTE.
The patentee can file a request for PTE for a patent when the relevant drug marketing authorization is approved. Note that the below conditions9 apply:
(1) The patent is granted before the drug marketing authorization application is approved;
(2) The patent is valid at the time of the PTE request;
(3) The patent has never received PTE;
(4) The claims in the patent comprise the technical solutions related to the new drug that has obtained marketing authorization;
(5) If a drug has multiple patents, PTE can only be requested for one of the patents;
(6) If a patent involves multiple drugs, PTE can only be requested for one drug.
As seen, the Examination Guideline imposes certain limitations on the request for PTE, limiting that a drug can only request PTE for one patent, a patent can only request PTE for one drug, and a patent can only receive PTE once.
The CNIPA provides the following examples10:
- if a drug a1 involves multiple patents b1, b2… bn, the patent holder of the drug can only request PTE for one of the patents (such as b1);
- a patent, such as b2, covers more than one drug (where each claim involves a different drug composition a1, a2… an); even though all of these drugs a1, a2… an have obtained market authorization from the NMPA, the patentee of patent b2 can only choose one of the drugs (such as a2) to request for PTE;
- when requesting for PTE, the same patent can only be extended once, or in other words, the same patent can only be “used” once.1
There are also certain procedural requirements.11 If the patentee and drug marketing authorization applicant are different parties, the patentee will need to submit the written approval of the drug marketing authorization applicant when requesting PTE. The patentee will also need to submit technical materials to prove that the drug approved is within the scope of the patent requesting PTE.
The patentee must file a request for PTE within 3 months from the date when drug marketing authorization is approved, and pay the relevant fee. Information regarding this PTE request fee has not published yet, but a new official fee schedule is expected to be released soon. According to an official announcement from the CNIPA on 18 Jan 2024,12 patentees who have already file a request will need to pay the fee after the new fee schedule is released.
Challenging a Decision
Once the Patent Office has made a decision to grant PTE, the relevant matters will be published on the patent registration book and the Patent Gazette.
If the Patent Office thinks that a patent is not eligible for PTE, it will issue a notice and the patentee will have a chance to response. After taking consideration of the patentee’s response, the Patent Office will issue a decision (of either granting a certain compensation term, or not granting any PTE). If a decision of not granting a PTE is issued, the Patentee may appeal via an administrative review with the CNIPA.1
To date, we have not seen any published patents having received any granted PTE.
EIP Thoughts
Although these rules are introduced based on the PTE systems in the US and Europe, several differences stand out that applicants should note.
PTE is only for “New” Drugs
First, PTE is only available for patents covering a “new drug”, which importantly refers to drugs that have not been marketed overseas or domestically. Many foreign companies apply for marketing authorization in their home countries first before entering the China market. Anyone who takes this regulatory route will likely not be eligible for PTE in China. The rules are clear that only innovative drugs (or certain improved drugs, e.g., prodrugs, new indications) that have never been marketed anywhere before, are eligible for PTE.
Foreign companies who wish to take advantage of PTE in China should strongly consider a regulatory pathway that involves applying for marketing authorization in China first, or at least simultaneously with their first marketing authorization application.
Limits on PTE Requests
Second, there appear to be limitations on the flexibility for requesting PTE. On the surface, both the US and China stipulate that a patent can only receive PTE once, and each drug can only receive PTE for one patent. However, China seems to add another limitation with respect to how many PTEs can be requested. Unlike in the US, where an applicant can make multiple PTE requests for the same drug in order to choose the “best” PTE decision, in China the plain language seems to indicate that a drug can only request PTE for one patent, and a patent can only request PTE for one drug. It’s not clear if you can request PTE again for a patent if it was rejected previously. We are trying to clarify some of these issues with the CNIPA/NMPA, and will update this article if we find out more clarity. For now, if a single patent might cover multiple different products, it’s best to consider a divisional strategy that separates the products into different divisional patents, allowing each product the chance to get its own PTE.
The Interplay of PTA and PTE
In both China and the US, PTA and PTE terms are “additive”. Though you need to request for each compensation right separately, once received you can add the two terms together to arrive at the final extension period, provided that the total extended time for PTE does not extend 5 years, and the total effective term of the patent after all extensions are added does not extend beyond 14 years from the marketing authorization date.
The PTE period will start after the PTA extension time is exhausted. It is also important to note that the protection scope is limited to the drug approved for marketing during the PTE period, while the PTA period covers the entire scope of the granted claims.
Furthermore, China’s PTE calculation method is basically identical to the European method of calculating SPC. It is different from the US method, which gives different weight to delays in the period between IND and NDA, and between NDA and the approval date.
Strategic Tips
As the implementation rules for PTE just came out at the end of 2023, it will take some time before we see how these rules really play out. At a minimum, we strongly recommend taking into consideration China’s PTE as part of an overall patent and regulatory strategy for China. As can be seen from the rules above, decisions such as where to conduct your first clinical trial, divisional filing strategy, what compounds/ products to include in a single patent, and other decisions can greatly impact the protection scope around a product in China.
As always, we will keep abreast of the latest developments and will write about them here, so please watch this space!
If you would like to have more information on this matter or would like to have our advice, please feel free to contact us at eip@eipgroup.asia.
Eagle IP is a top-tier boutique patent firm with a unique mix of experienced US and Chinese patent professionals with significant cross-border knowledge and experience. Our technically expertise covers wide range of technologies including, but not limited, to life sciences, biotechnology, medicine, pharmaceuticals, material and environmental science, chemistry and consumer electronics. We have years of experiences in drafting and prosecuting patent applications involving biological deposits, sequence listings, small and large molecules, drug discovery and development, material science, software and engineering, and many others.
This article is for general informational purposes only and should not be considered legal advice or a legal opinion on a specific set of facts.
About the Authors

Audrey Cheung is a qualified Chinese Patent Attorney at Eagle IP, a Boutique Patent Firm with offices in Hong Kong and Shenzhen.

Jennifer Che, J.D. is President & Managing Director and a US Patent Attorney at Eagle IP, a Boutique Patent Firm with offices in Hong Kong and Shenzhen.

Yolanda Wang is a Principal, Chinese Patent Attorney, and Chinese Patent Litigator at Eagle IP, a Boutique Patent Firm with offices in Hong Kong and Shenzhen.
Patent Examination Guidelines” (2023) Revised Interpretation (2), https://www.cnipa.gov.cn/art/2024/1/18/art_2199_189879.html
↩︎Rule 82: The compensation period shall be determined based on the number of days between the patent application date and the date when the new drug obtains marketing authorization in China minus 5 years, and shall be determined on the basis of compliance with the provisions of Article 42.3 of the Patent Law.
↩︎Rule 80: The invention patents related to new drugs mentioned in Article 42.3 of the Patent Law refer to new drug product patents, preparation method patents, and medical use patents that comply with regulations.
↩︎- ↩︎
- The NMPA has the following classification for Chemical Drugs, Biological Drugs and Traditional Chinese Medicines:

For complete definitions, please see the Chemical Drug Registration Classification and Application Document Requirements, https://www.nmpa.gov.cn/xxgk/ggtg/ypggtg/ypqtggtg/20200630180301525.html?type=pc&m=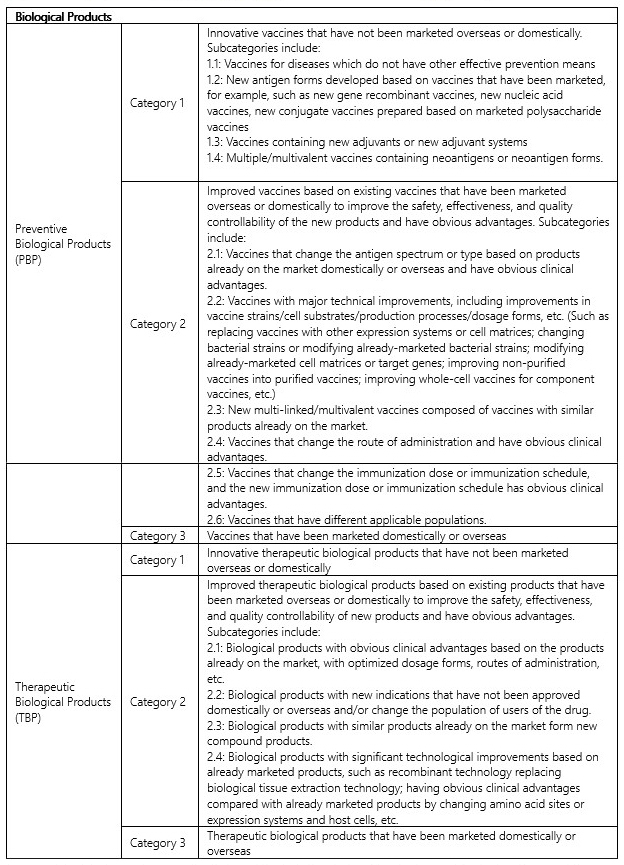
For complete definitions, please see the Biological Products Registration Classification and Application Document Requirements, https://www.nmpa.gov.cn/xxgk/ggtg/ypggtg/ypqtggtg/20200630175301552.html?type=pc&m=
For complete definitions, please see the Traditional Chinese Medicine Registration Classification and Application Document Requirements, https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/ggtg/ypggtg/ypqtggtg/20200928164311143.html ↑
↩︎ Preventive biological products refer to vaccine-type biological products used for human immunization to prevent and control the occurrence and spread of diseases, including immunization program vaccines and non-immunization program vaccines.
↩︎Therapeutic biological products refer to biological products used for the treatment of human diseases, such as proteins, peptides and their derivatives prepared using engineered cells (such as bacteria, yeast, insect, plant and mammalian cells) using different expression systems; cell therapy and gene therapy products; allergen original products; microbial products; biologically active products extracted from human or animal tissues or body fluids or prepared through fermentation, etc. Biological products in vivo diagnostic reagents are managed as therapeutic biological products.
↩︎Examination Guidelines Part 5 Ch.9 Section 3.4
↩︎Examination Guidelines Part 5 Ch.9 Sections 3.1
↩︎- https://www.cnipa.gov.cn/art/2024/1/18/art_2199_189879.html
↩︎ Examination Guidelines Part 5 Ch.9 Sections 3.3
↩︎Notice on the handling of patent term compensation, https://www.cnipa.gov.cn/art/2024/1/18/art_75_189871.html
↩︎





